Bone Growth Stimulator by Majestic DME
Bone Stimulator Insurance Commercial Coverage
To determine the cost of bone growth stimulators, review your specific insurance and verify what documentation you must provide to receive bone growth stimulator coverage. Depending on your insurance provider, the guidelines for determining whether a bone growth stimulation device is covered will differ. For instance, for individuals covered by Anthem BlueCross, the following conditions must be met before they will cover an electrical bone growth stimulator: at least 45 days have passed since the date of fracture or the date of surgical treatment of the fracture; serial radiographs or appropriate imaging studies were performed to confirm that no progressive signs of healing have occurred; and the fracture gap is less than 1 centimeter [3].
In order for these conditions to be officially met, you must provide documentation to your insurance provider. For example, if electrical or electromagnetic bone growth stimulation is required and the insurance provider is United Healthcare, you need to provide official medical notes documenting all of the following: 1) current physician prescription or order; 2) the reason a bone growth stimulator is needed; 3) any risk factors that apply (such as obesity or smoking) [4]. These requirements vary slightly from insurance provider to insurance provider and depend on the type of bone growth stimulant sought (electrical bone growth stimulator, ultrasonic bone growth stimulator, or electromagnetic bone growth stimulator). You can learn more about general insurance criteria for coverage for several providers here, including medicare guidelines for bone growth stimulators. Determining your health insurance coverage is essential in order to estimate bone stimulator costs.
All of the documentation requirements to gain approval for bone growth stimulators are already set by your insurance company. Most are available on the insurance company’s website. However, we are here to help. We work with health care providers that participate in all insurance programs to determine your documentation needs. Contact us today to determine if you qualify for a bone growth stimulator.
Coverage criteria for bone growth stimulators-
Your health insurance company may help cover the cost of a bone growth stimulation device. The first step is to determine the coverage criteria for your particular insurance company. You also have to decide which type of bone growth stimulator you are interested in as the type of bone growth stimulator will also affect criteria for insurance. Your insurance provider will also require documents providing proof of non-healing (usually 45 to 90 days of time) in order to qualify for coverage of a bone growth stimulator device.
For example, if electrical or electromagnetic bone growth stimulation is sought and the insurance provider is United Healthcare, the fracture must have 90 days of non-healing (nonunion) and you must provide official medical notes documenting all of the following: 1) current physician prescription; 2) documentation explaining the reason a bone growth stimulator is needed; 3) any risk factors that apply (such as obesity or smoking). Additionally, your insurance company will need medical office notes documenting: 1) date, site and type of fracture; 2) diagnostic imaging reports; 3) treatment of the fracture, including treatment already completed and treatment planned [5].
Patient pay and insurance estimates-
The out of pocket cost for a bone growth stimulator depends on your insurance provider. For individuals receiving Medicare benefits, the cost you will have to cover is generally 20% of the Medicare allowable amount. It is important to know that this technology is expensive, and the price of a bone growth stimulation unit can range from $500 to $5,000. Contact your insurance provider today to determine your exact patient pay and insurance estimates.
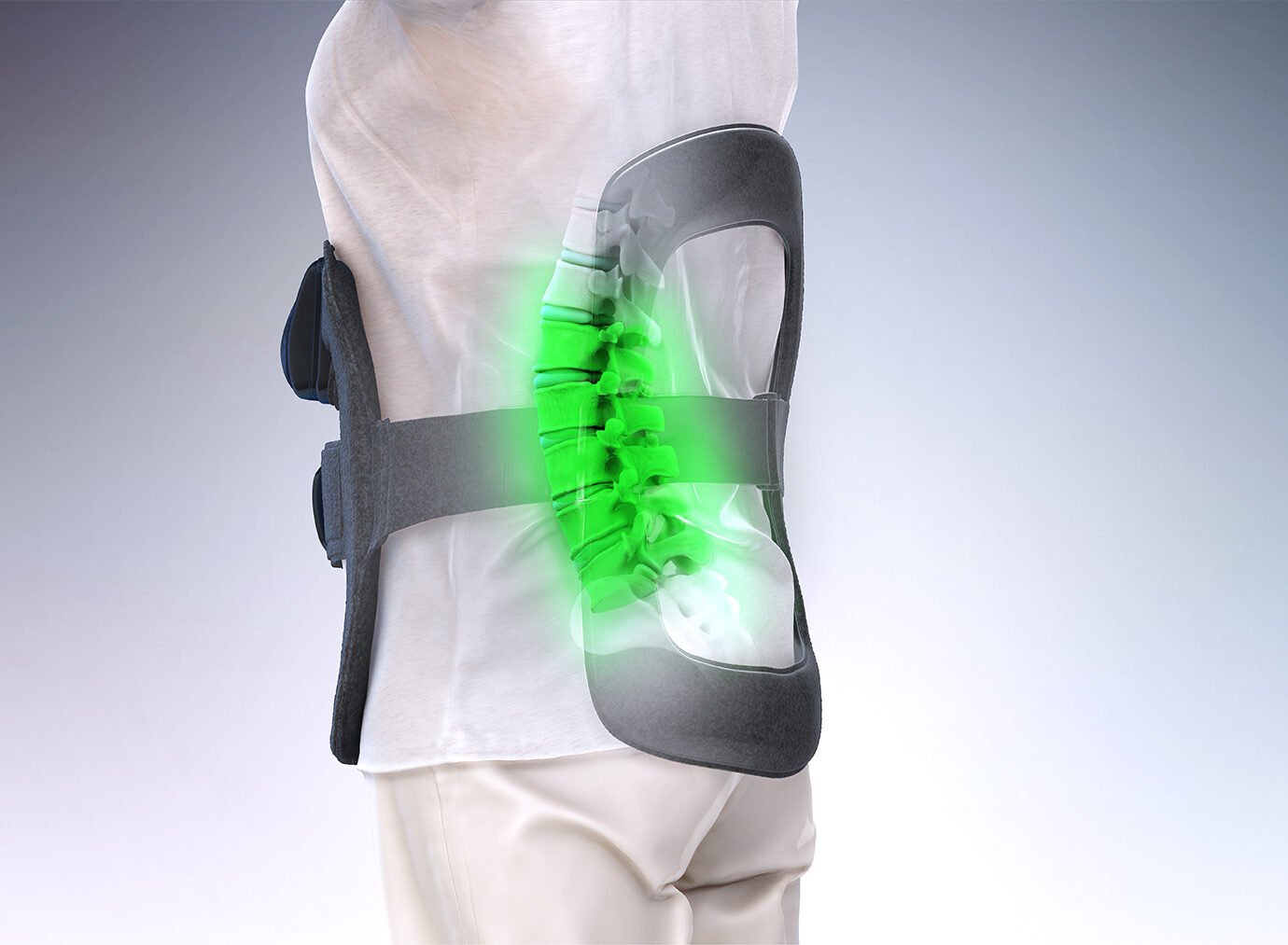
Orthofix Bone Growth Therapy devices provide a safe, noninvasive treatment that helps promote healing in fractured bones and spinal fusions that have not healed or have difficulty healing. These devices deliver energy to the healing bone using pulsed electromagnetic or ultrasound waves, which can significantly help the healing process.
Some patients face challenges in healing after a fracture or spinal fusion surgery. Factors like age, smoking, or certain medical conditions can slow the body’s natural bone-healing process. That’s where Bone Growth Therapy comes in.
Doctors often prescribe this safe, noninvasive treatment—also called bone growth stimulation—to support the healing process and improve outcomes in at-risk patients.
The CervicalStim device has been approved by the FDA to be worn after cervical spine fusion surgery in patients at risk for non-fusion.1,2 For complete prescribing information, please refer to the Instruction Manual.
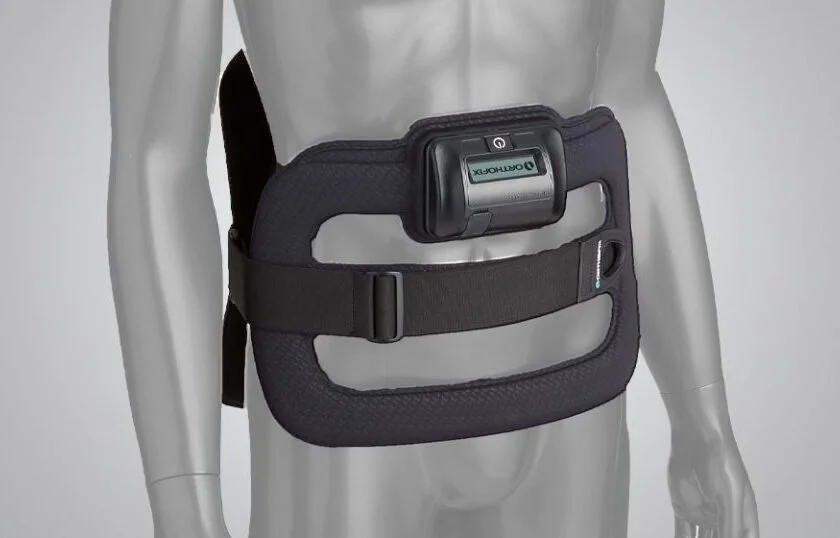
It is a single-piece device that is lightweight, flexible and portable, allowing freedom of movement during treatment. An LCD and audible alarm provide important feedback during treatment such as the operational status, treatment time remaining, battery capacity, etc.
Designed for patient ease of use
Works effectively when worn over clothing or bracing
Single-piece, cordless design allows for ease of placement and patient mobility
The STIM onTrack™mobile app is patient-friendly and provides patients with a treatment calendar, therapy reminders, and additional educational resources.*
We work with providers that participate in all insurance programs – contact us now to determine if you qualify for a bone growth stimulator.
Our team of insurance experts knows how to work with your insurance company to get your bone growth stimulator approved. We have in-network access to hundreds of insurance companies and thousands of plans. We work with companies such as: Aetna, Anthem, Blue Cross/Blue Shield, Cigna, Humana, Tri-Care, Worker’s Compensation, United Health Care, Department Of Labor OWCP Workers Compensation program, and many more throughout the US.
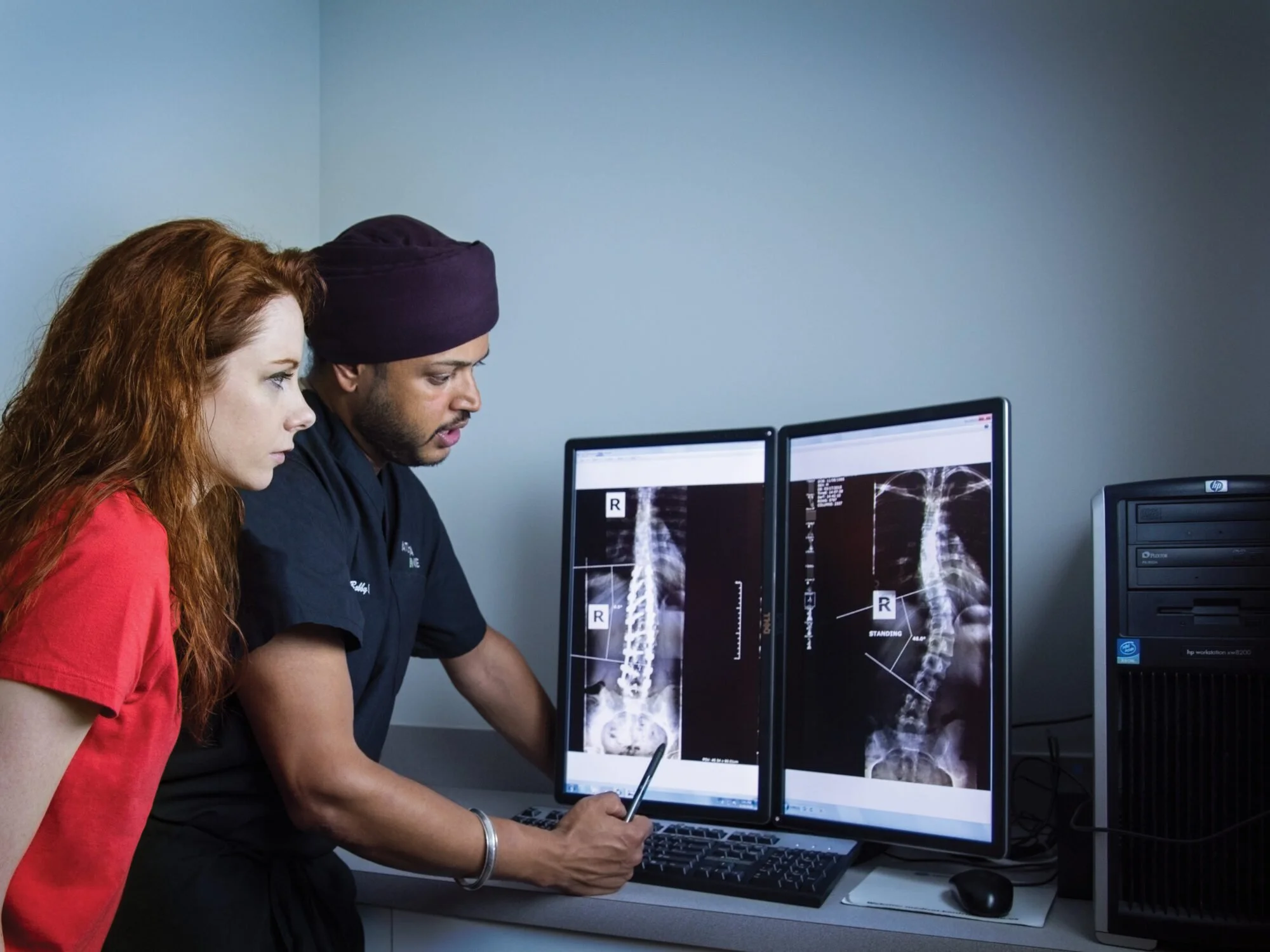
Coverage for a bone growth stimulator varies by insurance carrier. Two x-rays taken 90 days apart showing insufficient healing are generally required for insurance approval. Worker’s comp patients can generally be covered without waiting 90 days.
Bone Growth Stimulator – Purpose, Function, and Ordering for Personal Injury Cases
A bone growth stimulator is an advanced medical device designed to accelerate and support the natural healing process of bones following a fracture, surgery, or orthopedic injury. It works by delivering low-level electromagnetic or ultrasonic pulses to the affected area, stimulating the body’s natural cellular activity and encouraging new bone formation.
This technology is particularly beneficial for patients whose healing may be delayed due to factors such as diabetes, smoking, osteoporosis, or complex fractures. By enhancing the body’s biological response at the site of injury, bone growth stimulators can significantly reduce recovery time and improve overall bone strength and alignment, leading to better long-term outcomes.
In personal injury or workers’ compensation cases, a bone growth stimulator can be prescribed as part of a client’s medical treatment plan to facilitate recovery from fractures sustained in an accident. The device not only supports the healing process but can also serve as clinical evidence of the seriousness of the injury and the extent of medical intervention required.
How to Obtain a Bone Growth Stimulator for a Personal Injury Case
1. Medical Evaluation and Prescription
The first step begins with the treating orthopedic physician or surgeon. After assessing the patient’s injury, they determine whether the fracture is at risk of delayed healing or nonunion (failure to properly heal). If indicated, the physician writes a prescription for a bone growth stimulator.
2. Verification and Documentation
In a personal injury case, supporting medical documentation — including X-rays, clinical notes, and treatment records — is gathered to justify the medical necessity of the device. This documentation is essential for insurance authorization or for inclusion as part of a legal claim.
3. DME Provider Coordination
Once the prescription is obtained, a Durable Medical Equipment (DME) provider facilitates the process of delivering the bone growth stimulator. The provider verifies the patient’s insurance coverage, manages all necessary billing documentation, and ensures the device meets all FDA and compliance standards.
4. Delivery and Training
The patient receives the device either through a clinical setting or via home delivery. A DME specialist provides personalized instructions on proper usage, duration, and care, ensuring the client fully understands how to operate the device for optimal results.
5. Reporting and Follow-Up
Progress is monitored through periodic medical evaluations. Documentation of the patient’s improvement can also be used to support the injury claim by demonstrating ongoing medical necessity and progress toward recovery.
In essence, a bone growth stimulator is more than just a medical device — it is a vital tool in both the physical healing process and the legal recovery process for clients involved in personal injury cases. It provides measurable medical support, aids recovery, and strengthens the overall documentation of injury treatment, helping ensure that clients receive the care and consideration they rightfully deserve.
Patient Stories
Real Patients. Real Stories. Real Lives.
Each year, Majestic DME, LLC and Orthofix helps thousands of people throughout the world live mobile, active and fulfilling lives. Doctors look to us to provide regenerative and reconstructive solutions that will enhance the health and well-being of their patients who may be suffering from degenerative diseases, trauma, sports injuries, limb deformities and other medical conditions.